TL;DR
Researchers at UC San Francisco used CRISPR to convert patient-derived white fat into calorie-burning 'beige' fat that outcompetes tumors for nutrients in laboratory and mouse models. Implanted fat organoids suppressed multiple cancer types and were engineered to consume specific metabolites, suggesting a adaptable living-cell therapeutic approach.
What happened
Scientists at UCSF used CRISPR gene editing to activate dormant brown-fat programs in ordinary white adipose cells and produced highly metabolically active 'beige' fat. A key gene identified in the screen was UCP1; cells engineered to express this program consumed large amounts of nutrients in co-culture assays and caused cancer cells to die of nutrient deprivation in trans-well experiments. The team then formed fat organoids from engineered cells and implanted them alongside tumors in mice; these implants suppressed growth of several cancer types, including breast, pancreatic and prostate tumors, and in some experiments were effective even when implanted at sites distant from the tumor. The researchers also modified fat cells to preferentially consume metabolites used by specific cancers — for example, programming cells to take up uridine to target pancreatic tumors. Experiments included same-patient breast cancer tissues modified ex vivo and implanted in mice. The study appears Feb. 4 in Nature Biotechnology and was supported in part by NIH and other funders. The paper is dedicated to first author Hai Nguyen, who died in 2024.
Why it matters
- Offers a new form of living-cell therapy that leverages a tissue (fat) routinely obtained and reimplanted in clinical practice.
- Demonstrates a metabolic, non-toxic strategy that can suppress multiple cancer types by depriving them of nutrients rather than directly killing tumor cells.
- Shows potential adaptability: fat cells can be engineered to consume metabolites that specific cancers rely on.
- If translatable, the approach could reach hard-to-access tumors because implanted fat cells had effects even when located away from tumor sites.
- Fat cells are described as remaining localized and immunologically compatible, which may simplify safety considerations for autologous therapies.
Key facts
- The work was conducted at the University of California, San Francisco and published Feb. 4 in Nature Biotechnology.
- Researchers used CRISPR to activate brown-fat genes in white fat; UCP1 emerged as a central gene driving the beige, high-energy phenotype.
- Engineered beige fat reduced survival of multiple cancer cell lines in trans-well assays and suppressed breast, pancreatic and prostate tumors in mouse models.
- Fat organoids made from engineered cells were implanted in mice and outcompeted tumors for nutrients, causing cancer cell starvation.
- Same-patient breast fat and tumor tissue were used in experiments: patient-derived fat was modified and suppressed that patient’s cancer cells in culture and in mouse implants.
- Fat cells were programmed to preferentially consume specific nutrients — e.g., uridine — to match metabolic dependencies of certain cancers.
- Funding sources include NIH, the UCSF Sandler Program for Breakthrough Biomedical Research, the UCSF Living Therapeutics Initiative and the California Institute for Regenerative Medicine.
- Senior author Nadav Ahituv is a cofounder and scientific advisory board member of Regel Therapeutics; Ahituv has filed a patent application related to concepts in the manuscript and receives funding from BioMarin.
- The paper is dedicated to first author Hai Nguyen, a postdoctoral researcher who initiated the experiments and died in 2024.
What to watch next
- Whether these engineered fat-cell implants move into formal preclinical safety studies and then human clinical trials — not confirmed in the source.
- Long-term behavior and safety of implanted engineered fat in larger animal models, including whether cells remain localized — not confirmed in the source.
- Translation to hard-to-reach tumors such as glioblastoma: the authors suggested potential relevance but testing in brain cancer models is not reported in the source.
- Regulatory, manufacturing and autologous-cell therapy logistics needed to adapt liposuction-derived fat into a clinical therapy — not confirmed in the source.
Quick glossary
- Beige fat: A form of adipose tissue with increased mitochondrial activity that burns energy to produce heat, distinct from energy-storing white fat.
- CRISPR: A gene-editing technology used to activate, delete, or modify specific genes within cells.
- Fat organoid: A three-dimensional cluster of fat cells grown in the laboratory that models tissue structure and function for experiments or implants.
- UCP1: A mitochondrial protein associated with heat generation in brown and beige fat that increases cellular energy consumption.
- Autologous cell therapy: A treatment approach that uses a patient’s own cells, modified and returned to the body, to avoid immune rejection.
Reader FAQ
Have these engineered fat cells been tested in humans?
Not confirmed in the source.
How do the fat cells suppress tumors?
Engineered beige fat cells increase nutrient consumption (driven in part by UCP1), outcompeting nearby cancer cells and causing nutrient deprivation.
Are the fat cells safe once implanted?
The authors note fat cells tend to remain localized and are routinely used in plastic surgery, but formal long-term safety and clinical data are not reported in the source.
Could this treat many cancer types?
In the reported experiments the approach worked against several cancers in mice and cell cultures; broader clinical efficacy in humans is not confirmed in the source.
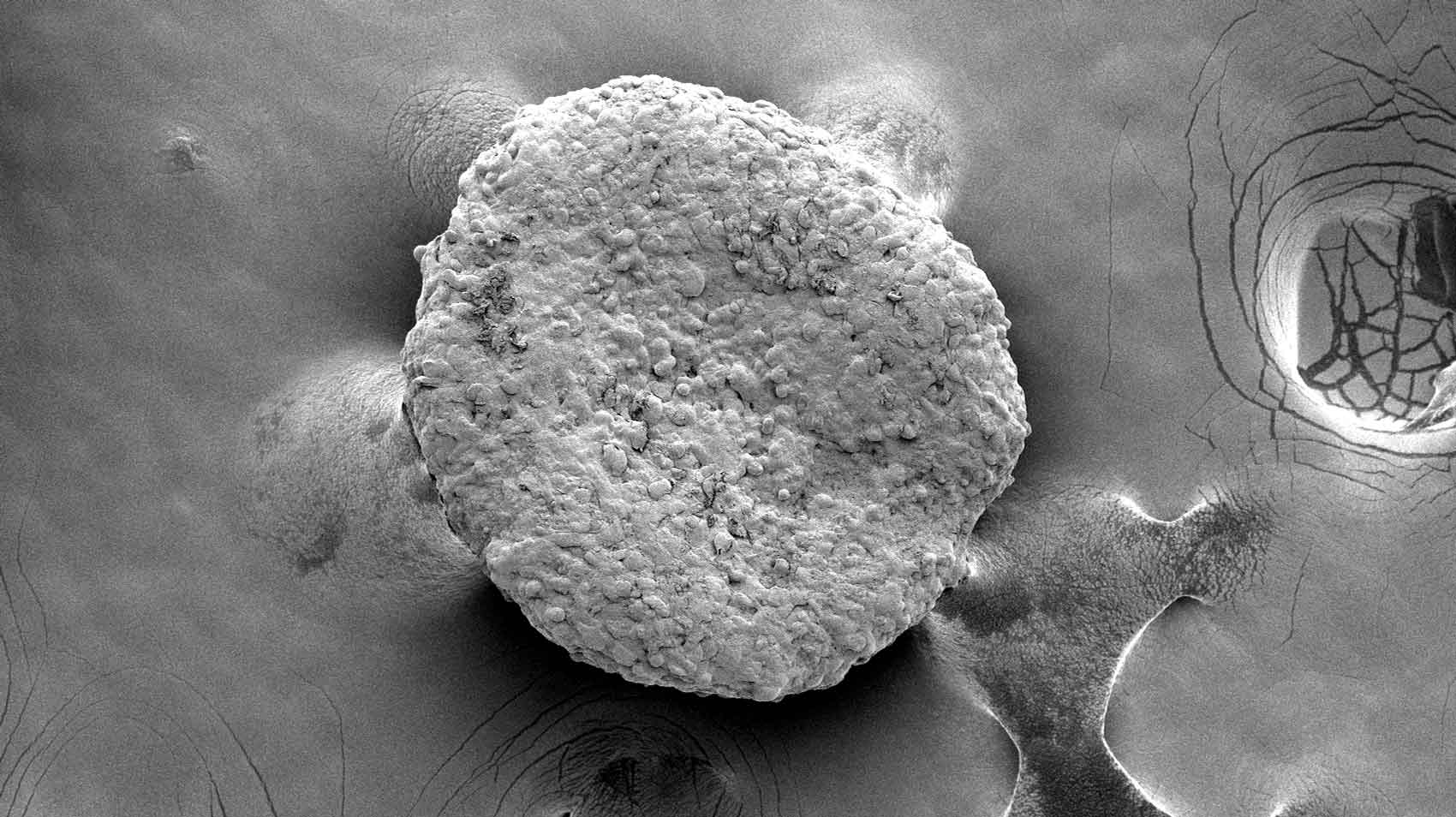
Research Dec. 22, 2025 UCSF Experts Present Research, Guidance at Hematology Meeting Electron microscopy of fat organoids that were implanted to out-compete tumors. Image by Desai Lab Research February 4,…
Sources
- Hungry Fat Cells Could Someday Starve Cancer to Death
- Genetically Modified Fat Cells Starve Tumors in Mice – NCI
- Implantation of engineered adipocytes suppresses tumor …
- Engineered Fat Cells Starve Cancer and Slow Tumor Growth
Related posts
- C++ says “We have try…finally at home” — cleanup via destructors and scope guards
- How Much Melatonin Should You Take? Guidance, Risks, and Alternatives
- Growing Up in 404: Life Inside China’s Secret Nuclear City in the Gobi