TL;DR
UCSF researchers used CRISPR to convert patient-derived white fat into calorie-consuming 'beige' fat that, when implanted, drew nutrients away from tumors and caused cancer cells to die in lab and animal tests. The approach worked against multiple cancer types in preclinical models and was reported in Nature Biotechnology.
What happened
Scientists at UC San Francisco edited white adipocytes to induce a beige, energy‑burning state and then tested whether those cells could outcompete cancer cells for nutrients. Using CRISPR activation of genes normally silent in white fat, the team identified UCP1 as a key driver and generated UCP1-expressing beige fat. In trans-well culture, beige fat separated from cancer cells but sharing media caused widespread cancer cell death across several lines. The group then implanted fat organoids next to tumors in mice and observed tumor suppression for breast, pancreatic and prostate models; implants placed away from tumors also reduced tumor growth. The researchers ran parallel experiments using human breast cancer samples and matched patient fat, and engineered fat to preferentially consume specific metabolites (for example, uridine) to match a tumor’s dietary dependence. The study, funded in part by the NIH, appears Feb. 4 in Nature Biotechnology and is dedicated to first author Hai Nguyen.
Why it matters
- Offers a novel living‑cell therapy strategy that deprives tumors of nutrients rather than directly targeting tumor cells.
- Uses tissue (fat) that is readily obtainable via routine procedures and can be expanded and modified ex vivo.
- Demonstrated activity across multiple cancer types and even at implantation sites distant from tumors, which could help reach hard-to-access cancers.
- The platform can be programmed to consume particular metabolites, suggesting adaptability to tumor-specific metabolic dependencies.
Key facts
- Researchers used CRISPR-based activation to convert white fat cells into beige-like, calorie-burning cells and identified UCP1 as a key gene.
- Initial trans-well experiments showed engineered beige fat caused extensive cancer cell death in multiple cell lines.
- Beige fat organoids implanted in mice suppressed breast, pancreatic and prostate tumors; distant implants also reduced tumor growth.
- Human breast cancer tissue from mastectomies was used in same-patient experiments; modified patient-derived fat outcompeted the tumor cells in culture and mouse models.
- The team engineered fat cells to preferentially consume specific nutrients (for example, uridine) to counter cancers with known metabolic preferences.
- The study was published Feb. 4 in Nature Biotechnology and received funding from NIH and UCSF programs.
- The paper is dedicated to Hai Nguyen, the first author, who moved to UT Austin in 2024 and died in November before completing final experiments.
- Disclosure: senior author Nadav Ahituv is a cofounder and on the scientific advisory board of Regel Therapeutics, has received industry funding, and has filed a patent application covering aspects of the work.
What to watch next
- Progression to human clinical trials and timelines for testing safety and efficacy in patients: not confirmed in the source.
- Regulatory and patent developments related to the patent application mentioned by the authors: not confirmed in the source.
- Further preclinical testing against additional tumor types and delivery sites — the authors suggested potential for hard-to-reach cancers like glioblastoma but clinical plans are not detailed in the source.
Quick glossary
- Beige fat: A type of adipose cell that, like brown fat, burns energy to produce heat rather than primarily storing calories.
- CRISPR activation: A gene‑editing approach that increases expression of specific genes rather than cutting DNA, used to switch on dormant pathways.
- Organoid: A three-dimensional cell culture that mimics the structure and function of real tissue and can be used for implantation or study.
- UCP1: A mitochondrial protein associated with heat generation in brown/beige fat that increases energy expenditure.
- Trans-well assay: A lab setup where two cell populations share media but are physically separated, allowing study of competition for nutrients or soluble signals.
Reader FAQ
Is this therapy ready for use in people?
Not confirmed in the source.
How were the fat cells converted to a calorie‑burning state?
Researchers used CRISPR-based activation to turn on genes normally active in brown fat; UCP1 was highlighted as a key effector.
Which cancers were affected in the experiments?
Preclinical work showed effects against breast, pancreatic and prostate cancer models, and lab tests included colon cancer lines as well.
Are there safety or migration concerns with implanting engineered fat?
Authors note fat cells tend to stay where implanted and have a long clinical history in plastic surgery, but formal human safety assessments are not reported in the source.
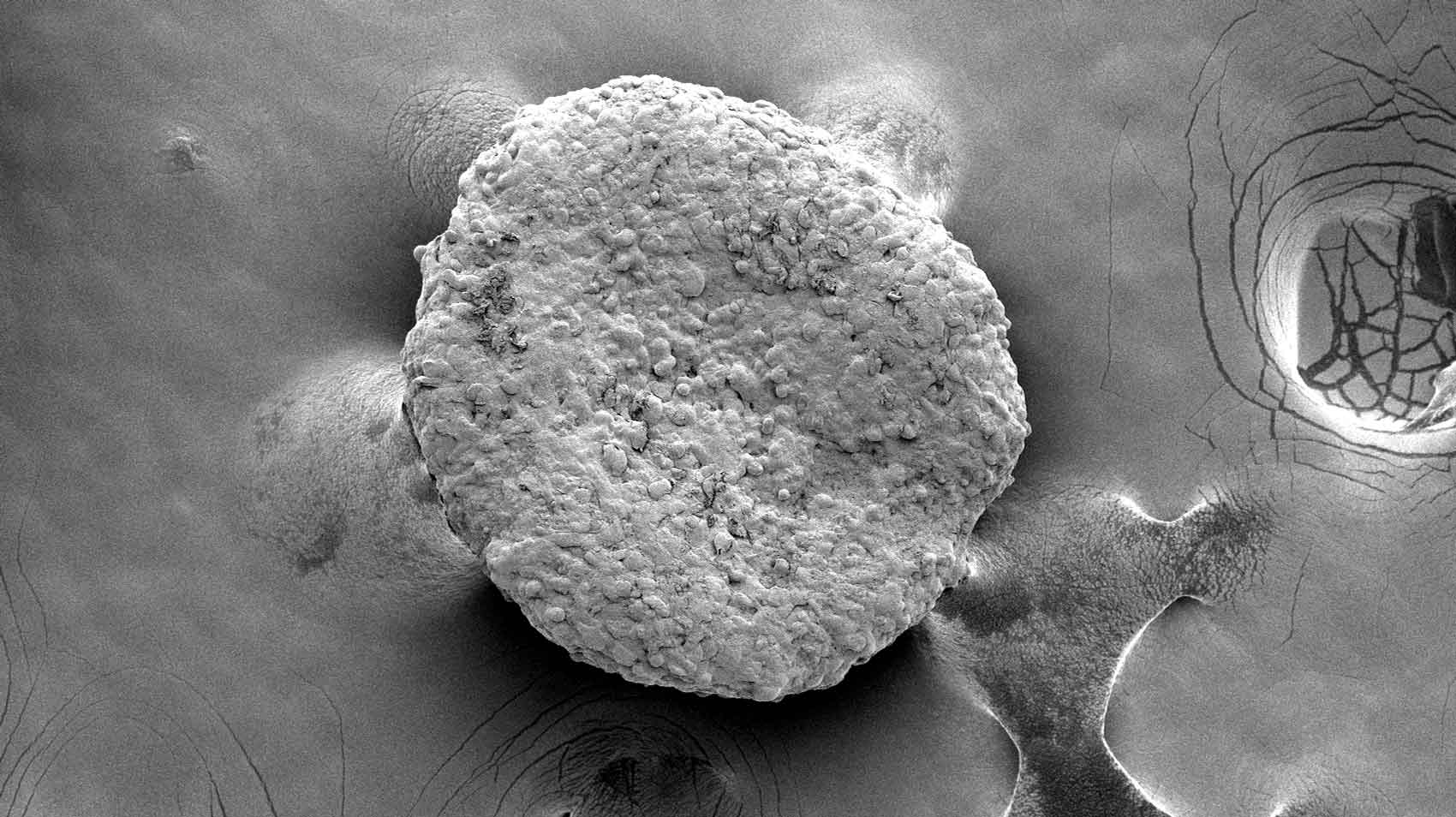
Research Dec. 22, 2025 UCSF Experts Present Research, Guidance at Hematology Meeting Electron microscopy of fat organoids that were implanted to out-compete tumors. Image by Desai Lab Research February 4,…
Sources
- Hungry Fat Cells Could Someday Starve Cancer
- Genetically Modified Fat Cells Starve Tumors in Mice – NCI
- Implantation of engineered adipocytes suppresses tumor …
- Engineered Fat Cells Starve Cancer and Slow Tumor Growth
Related posts
- Why ‘Deathbed Advice’ on Social Media Often Feels Cheap and Unhelpful
- Scratchapixel launches free, from-scratch computer graphics learning hub
- Two-thirds of Americans Expect AI to Cause Major Human Harm Within 20 Years